- 0 Comments
- PRMA Plastic Surgery
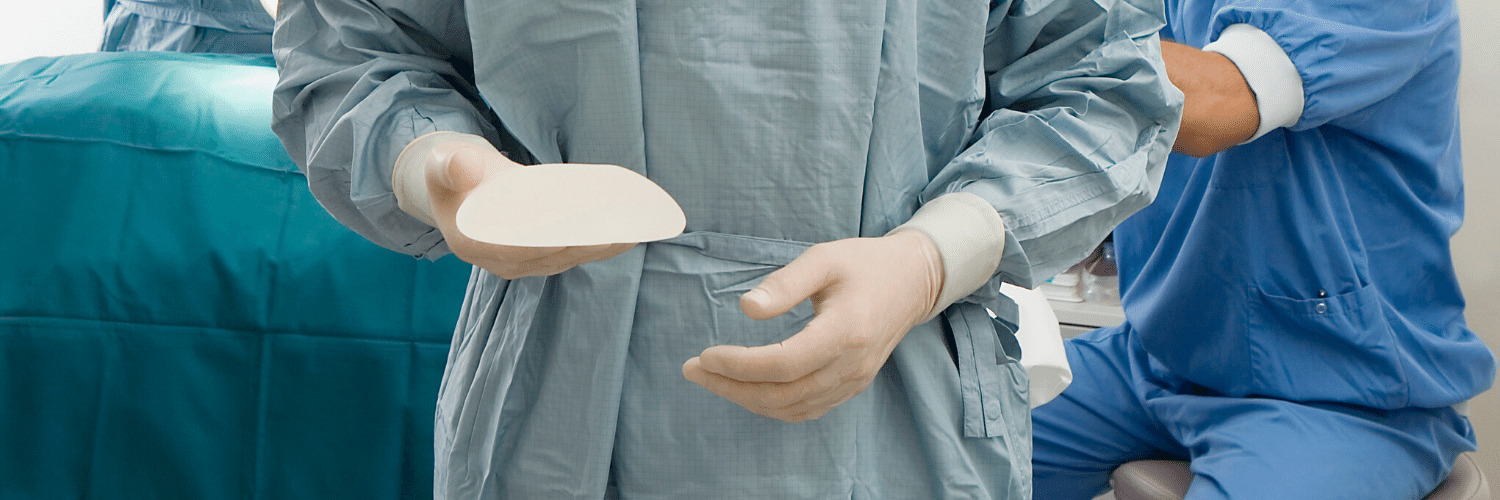
What is a capsular contracture?
Capsular contracture is the pathologic result of a chronic inflammatory process around a breast implant used for aesthetic or reconstructive purposes. Varying degrees of capsular contracture exist and can range from a mild form represented by minimal peri-prosthetic firmness and no subjective symptoms to a severe form that dramatically distorts the breast implant and is accompanied by persistent and significant breast pain. In order to better understand effective methods of prevention and treatment, the etiology of capsular contracture must be reviewed.
Recognized clinical causes of capsular contracture are indolent bacterial contamination (or biofilm), tissue trauma, hematoma, radiation, and silicone gel bleed. The pathologic process common to all these individual antecedent sources is the development of an inflammatory cell-mediated chronic inflammatory state around the breast implant. Each of the know sources of capsular contracture initiate a persistent and complex cascade of inflammatory cell infiltration around the implanted breast prosthesis.
Immediately following the introduction of an inflammatory stimulus around the breast implant such as each of the know causes of capsular contracture, inflammatory cells called neutrophils infiltrate the area of infection or injury with the purpose of neutralizing the stimuli with release of proteolytic enzymes. These powerful enzymes are designed to break down cellular particles in the inflammatory environment and initiate the reparative process. However, these power enzymes also have the capacity to injury normal tissue in a non-selective manner.
Once the reparative process has been initiated by neutrophils, a separate population of inflammatory cells called macrophages are subsequently recruited into the inflammatory site, not only to engulf and digest the remnants of inflammatory stimuli, but also to modulate the reparative process through release of growth factors and substances called chemokines that recruit other inflammatory cells as well as bone marrow-derived stem cells that will differentiate to form the foundation of healing tissue. Moreover, a critical function of macrophages is also to coordinate the efflux of neutrophils away from the site of inflammation when the stimulus is neutralized. If the inflammatory stimulus is minimal and completely eliminated, this coordinated cascade concludes with formation of a thin capsule around the breast implant devoid of any inflammatory cells. If neutrophils, however, remain in the tissue surrounding the implant due to ongoing stimuli, collateral damage to normal tissue continues perpetuating the inflammatory and reparative processes resulting in a thick and constricting capsule.
The constricting quality of capsular contracture has been a subject of research previously due to the presence of a unique cellular population within the capsule called myofibroblasts. These unique cells not only produce collagen molecules, which are the building blocks of the architectural scaffold where cells reside, but also have the capacity to contract much like normal muscle cells. Rows of linearly oriented myofibroblasts contract around an implant leading to shape distortion and tightness. The origin of the myofibroblast is an ongoing area of research but data suggest these cells are formed from bone marrow derived mesenchymal stem cells that are induced to differentiate by macrophage-produced cytokines and growth factors.
Author: Dr. Oscar Ochoa
Capsular contracture is the pathologic result of a chronic inflammatory process around a breast implant used for aesthetic or reconstructive purposes.
Leave Comment
Sign Up for Our Monthly Newsletter
Continue Reading

Capsular Contracture Part One: What is it?

Prophylactic Double Mastectomy with Immediate DIEP Flap Breast Reconstruction: Dvora’s Experience
Prophylactic Double Mastectomy with Immediate DIEP Flap Breast Reconstruction: Dvora’s Experience July 16, 2020 Share on Facebook Twitter Linkedin Is DIEP flap reconstruction an option after prophylactic mastectomy? Due to a high risk of breast cancer, Dvora chose to undergo bilateral prophylactic nipple-sparing mastectomy with immediate DIEP flap breast reconstruction. “The minute I found out I was BRCA1 positive, […]

Tissue Expander/Implant vs DIEP Flap Breast Reconstruction: Which Provides the Easiest Recovery?
Implant vs DIEP Flap Breast Reconstruction: Which Provides the Easiest Recovery? July 16, 2020 Share on Facebook Twitter Linkedin What breast reconstruction procedure has the easiest recovery? When considering breast reconstruction, a big concern for many patients is post-operative pain. Many women presume breast reconstruction with implants is the least painful option since the procedure […]

Male Breast Cancer Survivor Strives to Bring Awareness to the Disease
Male Breast Cancer Survivor Strives to Bring Awareness to the Disease July 16, 2020 Share on Facebook Twitter Linkedin What is is like having male breast cancer? Recently I had the privilege of speaking with a lovely couple whose lives’ have been impacted by breast cancer. This is their story: A few years back, Michael […]

Helping Women Restore Feeling To Reconstructed Breast After Mastectomy
Helping Women Restore Feeling To Reconstructed Breast After Mastectomy July 16, 2020 Share on Facebook Twitter Linkedin How do surgeons help restore feeling after a mastectomy? Breast reconstruction helps women feel “whole” again both physically and emotionally after breast cancer by restoring what cancer tried to take away. During a mastectomy, nerves that supply the […]

10 Tips to Prepare for Breast Reconstruction Surgery
10 Tips to Prepare for Breast Reconstruction Surgery July 16, 2020 Share on Facebook Twitter Linkedin How can you prepare for breast reconstruction surgery? If you were going to give a close friend one piece of advice to prepare for breast reconstruction, what would it be? This is the question I recently presented to our social […]
DIEP Flap Breast Reconstruction on the Rise
DIEP Flap Breast Reconstruction on the Rise July 16, 2020 Share on Facebook Twitter Linkedin How often is DIEP flap reconstruction performed in the nation? A recent study published by PubMed shows there is an increased use of autologous breast reconstruction after mastectomy. Using the Nationwide Inpatient Sample database, information on breast cancer and mastectomy […]

PRMA is Celebrating 607 DIEP Flaps for 2016
PRMA is Celebrating 607 DIEP Flaps for 2016 July 16, 2020 Share on Facebook Twitter Linkedin A few weeks ago, we announced our record breaking 600th DIEP flap breast reconstruction procedure for 2016! We kept at it for the few working days remaining in the year, and we are proud to announce we performed a […]

PRMA Plastic Surgery Represented at ASRM 2017
PRMA Plastic Surgery Represented at ASRM 2017 July 16, 2020 Share on Facebook Twitter Linkedin The American Society of Reconstructive Microsurgery held its annual meeting in Hawaii this year! The meeting kicked off last Thursday and wraps up today! The American Society for Reconstructive Microsurgery (ASRM) was established in 1984 and has served “to promote, […]
Breast Cancer “Vaccine” Offers Hope
Breast Cancer “Vaccine” Offers Hope July 16, 2020 Share on Facebook Twitter Linkedin What is the breast cancer vaccine? Researchers have announced a potential new treatment for early stage breast cancer. The therapy is being called an “immunology vaccine” and works by enhancing your immune system, helping your body to use its best weapon to […]

No Comments