- 0 Comments
- PRMA Plastic Surgery
March 26th, 2019 concluded the US Food and Drug Administration’s two-day hearing reviewing the safety of breast implants.
The hearing included breast implant manufacturers, physicians and patients who have experienced health complications they believe are directly linked to their implants. Some of the described conditions included chronic fatigue, hair loss, joint pain and headaches—collectively known as “breast implant illness.” The most alarming complication mentioned was cancer.
Although there has not yet been any scientific evidence to prove implants are the cause of “breast implant illness,” there is evidence to support that textured implants can cause a rare form of cancer known as Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL).
About 300,000 breast augmentations and 100,000 implant-based breast reconstructions occur in the U.S. each year, according to the American Society of Plastic Surgeons. To date, over 660 women have been diagnosed with BIA-ALCL with a reported 12 deaths.
Some of the patient testimonies during the hearing called for a complete ban of textured breast implants. However, the FDA did not recommend any immediate restrictions at this time, stating that more information would be needed to fully understand the causes of the implant related health problems. In fact, later this summer, the US National Breast Implant Registry is expected to launch, providing a national database to collect real time data on patients diagnosed with BIA-ALCL to better understand this rare complication.
The FDA did however strike Mentor and Sientra (manufactures of breast implants) with warning letters last week for failing to fully meet post-market study requirements. Mentor cited falling rates of textured implant use as a challenge, while Sientra said they faced proper study issues due to women healthy and satisfied with their implants not participating in follow-up studies.
A common agreement throughout the hearing was the need for better education and surveillance of breast implant related health problems. Patients consistently reported not being informed of the potential risks associated with breast implants or the potential need for additional surgeries down the road. Some patients testified they felt “blindsided” by their doctors and called for new legislation on informed consents.
In conclusion, textured implants are likely not going off the US market anytime soon, even though they have been pulled from the shelves in Europe. We can be encouraged however in knowing that patient and physician advocates are making an impact and their voices are being heard. With calls for more research and better patient education, we can have hope that there will be better understanding of implant’s long-term impact on patient health and improved patient education.
Author: Dr. Minas Chrysopoulo and Courtney Floyd
A common agreement throughout the hearing was the need for better education and surveillance of breast implant related health problems.
Leave Comment
Sign Up for Our Monthly Newsletter
Continue Reading

Breast Reconstruction Surgery and Your Period
Breast Reconstruction Surgery and Your Period December 02, 2020 Share on Facebook Twitter Linkedin During our pre-operative appointment with patients, a topic that occasionally arises is menstrual cycles. It is completely normal for women to experience changes with their periods throughout breast cancer treatments. These can be temporary or permanent. Periods can be unpredictable following […]

What Bras and Abdominal Girdles to Wear After Breast Reconstruction Surgery
What Bras and Abdominal Girdles to Wear After Breast Reconstruction Surgery November 17, 2020 Share on Facebook Twitter Linkedin When preparing for breast reconstruction surgery, many patients want to know what types of bras and abdominal girdles they should plan to wear after surgery. Although every surgeon has slightly different preferences, we have put together […]

My 5 DIEP Flap Realities | A Guest Blog From Julie
My 5 DIEP Flap Realities October 28, 2020 Share on Facebook Twitter Linkedin Hi everyone, my name is Julie from It’s a Bosom Thing. I am so happy to be here as a guest blogger and have this opportunity to share with you a few thoughts about life after DIEP Flap Surgery. I was diagnosed […]

PRMA’s BRA Day Virtual Event Recap
PRMA’s BRA Day Virtual Event Recap September 08, 2020 Share on Facebook Twitter Linkedin Yesterday we celebrated Breast Reconstruction Awareness day! Although we missed seeing everyone in person this year, we were still able to spread education and awareness on ALL reconstructive options through our virtual efforts. We were also able to share information on […]
Second Stage DIEP Flap Surgery
Second Stage DIEP Flap Surgery September 08, 2020 Share on Facebook Twitter Linkedin DIEP flap breast reconstruction is typically comprised of at least two stages for the best outcomes. The second stage of surgery is commonly referred to as the “revision” stage and is usually performed about three months after the initial reconstruction. The purpose […]

If ‘Flaps’ Are Such A Great Breast Reconstruction Option, Why Doesn’t Everyone Get Them?
If ‘Flaps’ Are Such A Great Breast Reconstruction Option, Why Doesn’t Everyone Get Them? September 08, 2020 Share on Facebook Twitter Linkedin Flap-based breast reconstruction procedures, like the DIEP flap, offer patients a safe, natural implant-alternative option to reconstruction after a mastectomy. Flap surgeries are permanent and are associated with fewer complications after radiation when […]
What is a Skin Island and How is it Used in Breast Reconstruction?
What is a Skin Island and How is it Used in Breast Reconstruction? September 08, 2020 Share on Facebook Twitter Linkedin What is a “skin island”? The term “skin island” is used to describe the remaining visible skin from a transplanted “flap” of tissue. In the setting of DIEP flap breast reconstruction, the skin island […]
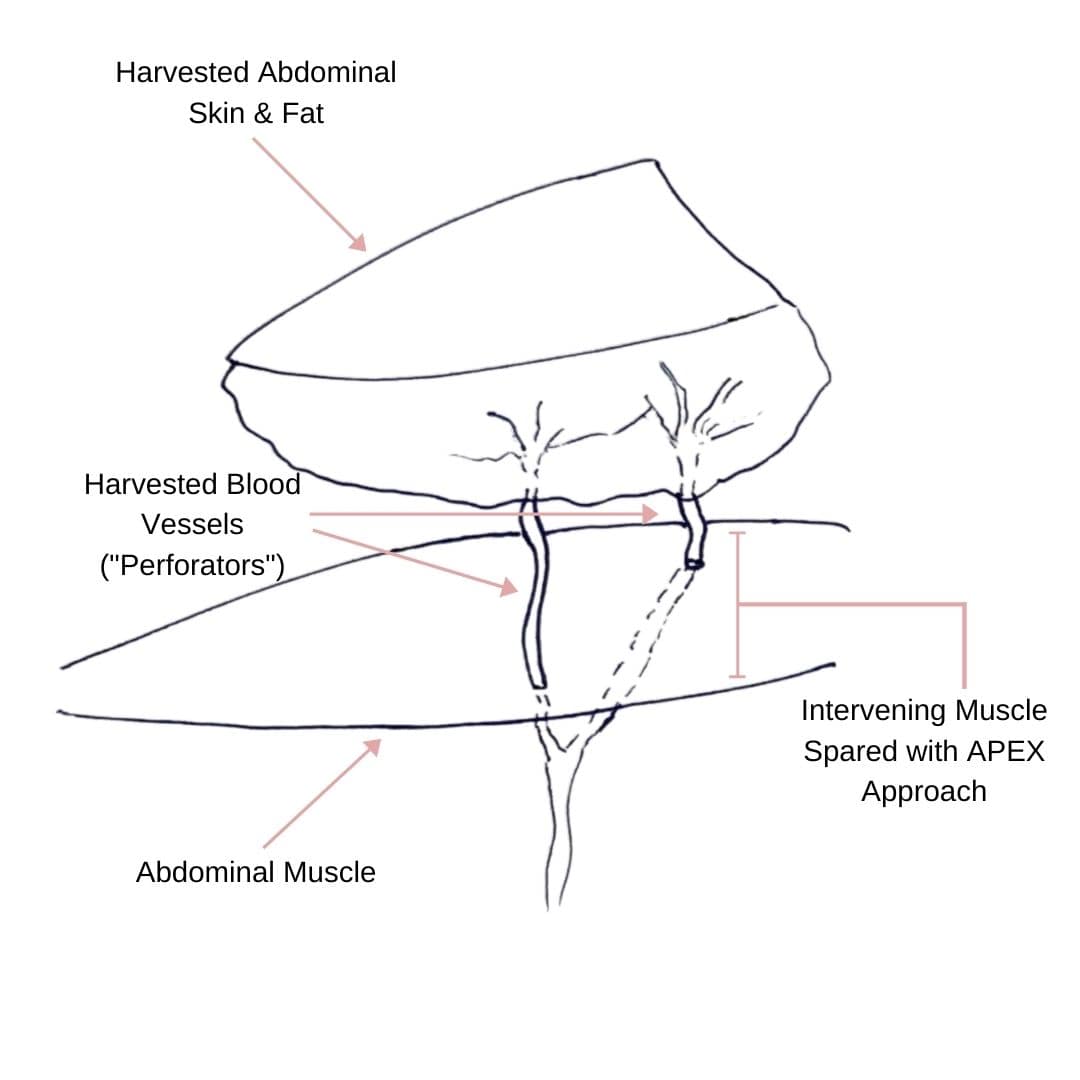
Comparing APEX Flap and DIEP Flap Breast Reconstruction
Comparing APEX Flap and DIEP Flap Breast Reconstruction August 10, 2020 Share on Facebook Twitter Linkedin We have been receiving numerous inquiries about the “APEX flap” recently. Patients want to know what it is and how it differs from the DIEP flap. APEX is an acronym that stands for “Abdominal Perforator Exchange”. Many patients believe […]
Monitoring the Health of Your Flap During & After Surgery
Monitoring the Health of Your Flap During & After Surgery July 21, 2020 Share on Facebook Twitter Linkedin Autologous flap (or tissue) breast reconstruction procedures represent today’s most advanced options for rebuilding a breast(s) following mastectomy. The most commonly performed method of flap-based reconstruction at PRMA is the DIEP flap. During this procedure, surgeons transplant skin […]
COVID-19 and the Impact on Cancer Patient’s Mortality
COVID-19 and the Impact on Cancer Patient’s Mortality July 21, 2020 Share on Facebook Twitter Linkedin There is still so much we do not know about COVID-19. Likewise, there is little known about how this disease impacts mortality for cancer patients. A study published in The Lancet evaluated and characterized the outcomes of patients with cancer […]

No Comments